Prepare for a Challenging Flu Season with Fast, Multiplex Respiratory Testing
Find Out How One Lab Delivers Fast Respiratory
Results for Senior Care Populations
Too often, doctors ordering tests and patients worried about their health have to wait for clinical results. These delays can significantly impact a patient’s outcome, depriving doctors of the information they need to make the best therapeutic decisions for their patients.
A patient could have the flu, a bacterial infection, or may even have been exposed to COVID-19. How can labs get the results that doctors and patients need for effective treatment?
According to Steve Hoover, President of Lab Operations at Mako Medical, “We believe in syndromic testing because we don’t have to spend a lot of time diagnosing a patient who might not have a lot of time to get that diagnosis.”

While the team at Mako Medical described their experience with the QIAstat-Dx Respiratory Panel, you can expect a similar experience with the QIAstat-Dx Respiratory SARS-CoV-2 Panel, which provides results for 21 respiratory targets in about an hour. With this single-step syndromic test, your lab can prepare for the increased demand this flu season could bring.

New flyer: Co-infection in COVID-19 patients
A recent study has detailed the rates of co-infection in COVID-19 patients, highlighting the importance of multiplex testing for accurate diagnosis.
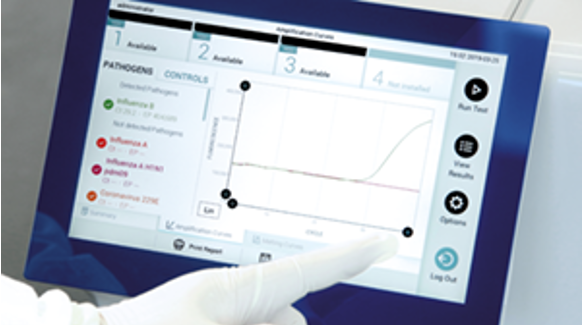
Learn more about QIAstat-Dx
Visit QIAstat-Dx.com to download our brochure, hear about more customer experiences, and learn more about our respiratory panel with SARS-CoV-2.
If you are located in the US, visit the QIAstat-Dx Respiratory SARS-CoV-2 Panel product page here: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/infectious-disease/qiastat-dx-syndromic-testing/qiastat-dx-eua-us/?clear=true#orderinginformation
If you are located outside the US, please visit QIAGEN.com to learn about solutions available in your region: https://www.qiagen.com/
The QIAstat-Dx Respiratory SARS-CoV-2 Panel is intended for in vitro diagnostic use under Emergency Use Authorization (EUA) Only. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity and moderate complexity tests.
The QIAstat-Dx Respiratory SARS-CoV-2 Panel has not been FDA cleared or approved.
The QIAstat-Dx Respiratory SARS-CoV-2 Panel has been authorized by FDA under an EUA for use by authorized laboratories.
The QIAstat-Dx Respiratory SARS-CoV-2 Panel has been authorized only for the detection and differentiation of nucleic acid of SARS-CoV-2 from multiple respiratory viral and bacterial organisms.
The QIAstat-Dx Respiratory SARS-CoV-2 Panel is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Want More?
Sign up for the GSS Resources Newsletter, an eNewsletter that delivers new technology and news, straight to your inbox.
GSS-Sales@govsci.com | 800.248-8030 | govsci.com

ISO 9001:2015 Certified
Copyright 2020, Government Scientific Source. All Rights Reserved.







